
- #DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SOFTWARE#
- #DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SERIES#
- #DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SIMULATOR#
Residue Curve Maps and Distillation Region Diagrams. Thermally Coupled Systems and Dividing Wall Columns. ĭISTILLATION SYSTEMS Possible Configurations of Distillation Columns. Synthesis of Multicomponent Separation Systems. Mechanical Design and Implementation Issues. Simulation, Modeling, and Design Feasibility. Extractive Distillation Design and Optimization. Solvent Effects in Extractive Distillation. Design and Operation of Azeotropic Distillation Columns. Exploiting Azeotropy and Liquid-Phase Immiscibility.
#DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SOFTWARE#
Software for Distillation Column Simulations. Example 14: The Need for Rigorous Maxwell-Stefan-Based NEQ Models. Example 13: A Nonequilibrium Model of a C4 Splitter. The equilibrium K factors can be estimated using the De Priester charts Dadyburjor (1978) Ans: bubble point 120 C, dew point 137 CQ2)The feed to. Example 12: Mass-Transfer Coefficients in a Packed Column. Example 11: Mass-Transfer Coefficient in a Tray Column. Example 10: Multiple Steady States in Distillation.
#DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SIMULATOR#
Using a Simulator to Solve Distillation Problems. Barnicki, authors of this section in the 7th edition. * Certain portions of this section draw heavily on the work of J. Example 8: The Industrial i-Butane/n-Butane Fractionator (Again). Example 7: An Industrial i-Butane/n-Butane Fractionator. Example 4: Light Hydrocarbon Distillation. Continuation Methods (for Really Difficult Problems). The Depriester charts (see Figures 4 and 5) are used to compute dew-point pressures, bubble point pressures, vapor-phase compositions, and liquid-phase compositions for hydrocarbons in the design of a flash drum. Degrees-of-Freedom Analysis and Problem Formulation. The MESH Equations (The 2c + 3 Formulation). Date, 11 December, Source, Chemical Engineering Symposium.
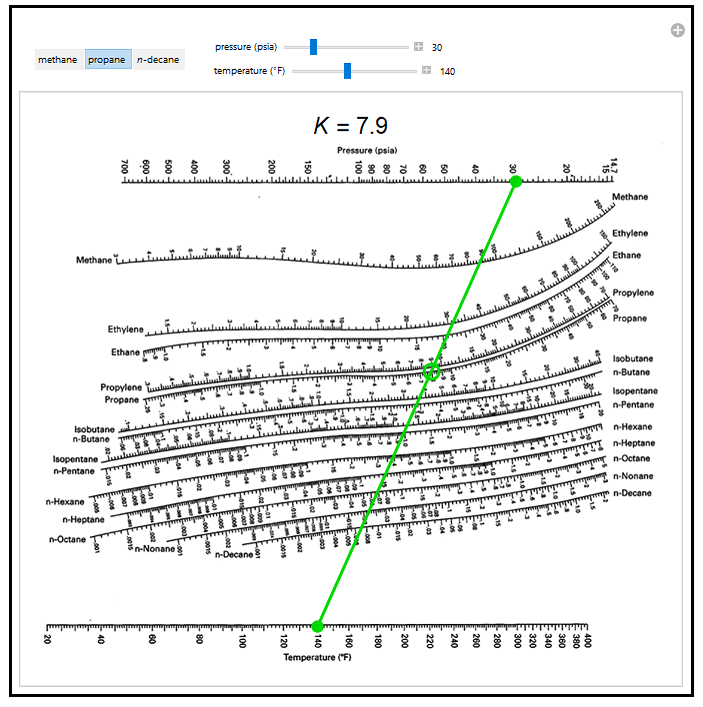

English: K-Values for systems of light hydrocarbons, high temperature range. 13-28 SIMULATION OF DISTILLATION PROCESSES Equilibrium-Stage Modeling. This Demonstration applies a DePriester chart, a set of nomograms, to find the vapor-liquid equilibrium ratio (the gas phase mole fraction divided by the liquid. 13-28 Example 2: Calculation of Kremser Method. 13-25 Example 1: Calculation of FUG Method. ĪPPROXIMATE MULTICOMPONENT DISTILLATION METHODS Fenske-Underwood-Gilliland (FUG) Shortcut Method. GRAPHICAL METHODS FOR BINARY DISTILLATION Phase Equilibrium Diagrams. SINGLE-STAGE EQUILIBRIUM FLASH CALCULATIONS Bubble Point and Dew Point. THERMODYNAMIC DATA AND MODELS Phase Equilibrium Data. Equilibrium and Nonequilibrium-Stage Concepts. INTRODUCTION TO DISTILLATION OPERATIONS General Principles. Professor of Chemical Engineering, Clarkson University (Simulation of Distillation Processes) Professor of Chemical Engineering and Dean of Engineering, University of Massachusetts-Amherst (Batch Distillation) R. Process Engineer, Air Products and Chemicals Inc. Professor of Chemical Engineering, University of California-Santa Barbara (Section Editor) Z. The dew point is estimated by iterative methods selecting K values at different assumed dew point (usually P or T are known) conditions, using y i/K i, the sum of which must equal 1 for the right (P,T) dew point selection.M. Sandler in Foundations of Computer Aided Design Vol2, p.83 (AIChE, 1981), may be used as practical and reasonable approximations.
#DEPRIESTER CHART FOR HYDROCARBONS TO FIND DEW TEMP SERIES#
However, in case of light hydrocarbons, the use of K i=y i/x i values taken from DePriester's graphs published by CEP Symposium Series No.7, vol.49, p.41, 1953, re-published in SI units in the CEP vol.74,(4), pp.85-86, April, 1978, or estimated by formulas as recommended by S.I. I must agree with him/her in that programs like the one he/she suggests are available, useful, and sometimes the only chance to reliably estimate VLE conditions for a mixture of chemicals.

To lad圜R, mirchee's approach using computer programs is known to me from another thread.


 0 kommentar(er)
0 kommentar(er)
